carbon
Carbon (from Latin: carbo "coal") is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. 碳是一种化学元素,符号为C,原子序数为6。它是非金属和四价的,使四个电子可以形成共价化学键。它属于周期表的第14组。碳只占地壳的0.025%。三种同位素自然产生,12C和13C是稳定的,而14C是放射性核素,半衰期约为5730年。碳是自古以来为数不多的已知元素之一。
Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
The atoms of carbon can bond together in diverse ways, resulting in various allotropes [同素异形体] of carbon. The best known allotropes are graphite, diamond, and buckminsterfullerene [巴克敏斯特富勒烯,巴克球,碳六十(碳的同素异形体,呈空心球状结构)]. The physical properties of carbon vary widely with the allotropic form. For example, graphite is opaque and black while diamond is highly transparent. Graphite is soft enough to form a streak on paper (hence its name, from the Greek verb "γράφειν" which means "to write"), while diamond is the hardest naturally occurring material known. Graphite is a good electrical conductor while diamond has a low electrical conductivity. Under normal conditions, diamond, carbon nanotubes, and graphene [石墨烯(由碳原子构成的单层片状结构的纳米材料)] have the highest thermal conductivities of all known materials. All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable form at standard temperature and pressure. They are chemically resistant and require high temperature to react even with oxygen.
Carbon forms a vast number of compounds, more than any other element, with almost ten million compounds described to date, and yet that number is but a fraction of the number of theoretically possible compounds under standard conditions. For this reason, carbon has often been referred to as the "king of the elements".
Some allotropes of carbon: a) diamond; b) graphite; c) lonsdaleite [六方金刚石]; d–f) fullerenes [富勒烯] (C60, C540, C70); g) amorphous carbon [非晶碳;无定形碳]; h) carbon nanotube
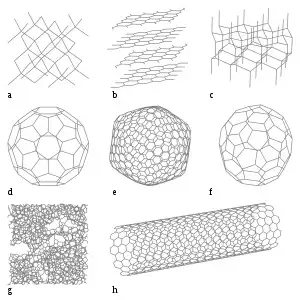
Carbon is the fourth most abundant chemical element in the observable universe by mass after hydrogen, helium, and oxygen.
Based on preliminary analysis, the global average atmospheric carbon dioxide in 2020 was 412.5 parts per million (ppm for short), setting a new record high amount despite the economic slowdown due to the COVID-19 pandemic.
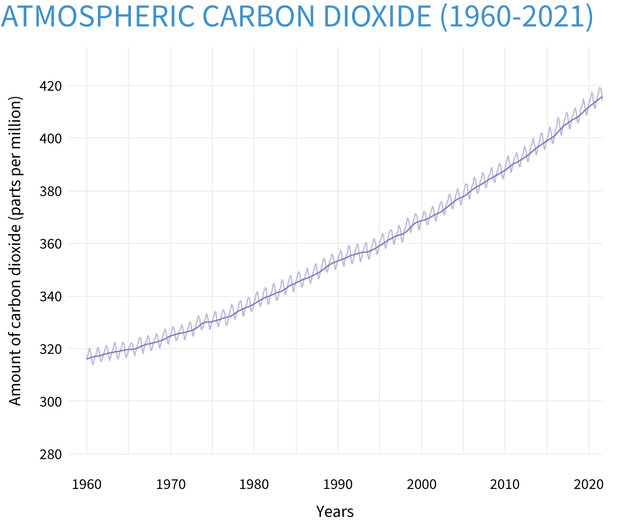
Climate Change: Atmospheric Carbon Dioxide | NOAA Climate.gov
六级/考研单词: carbon, electron, crust, decay, abundant, hydrogen, oxygen, diverse, organism, compound, encounter, atom, diamond, physics, opaque, transparent, streak, verb, conduct, norm, thermal, million, fraction, seldom, data, preliminary, globe, dioxide, despite




 浙公网安备 33010602011771号
浙公网安备 33010602011771号