CHM1001 学习笔记
| Word | Translation |
|---|---|
| isotope | 同位素 |
| fission | 裂变 |
| fusion | 聚变 |
| crystallization | 结晶 |
| dissolution | 溶解 |
| concentration | 浓度 |
| solubility | 溶解度 |
| (super)saturated | (过)饱和的 |
| (non)electrolyte | (非)电解质 |
| colloid | 胶体 |
| osmotic pressure | 渗透压 |
| activation energy | 活化能 |
| collide | 碰撞 |
| catalyst | 催化剂 |
| cease | 停止 |
| aqueous solution | 水溶液 |
| glucose | 葡萄糖 |
| stoichiometry | 化学计量数 |
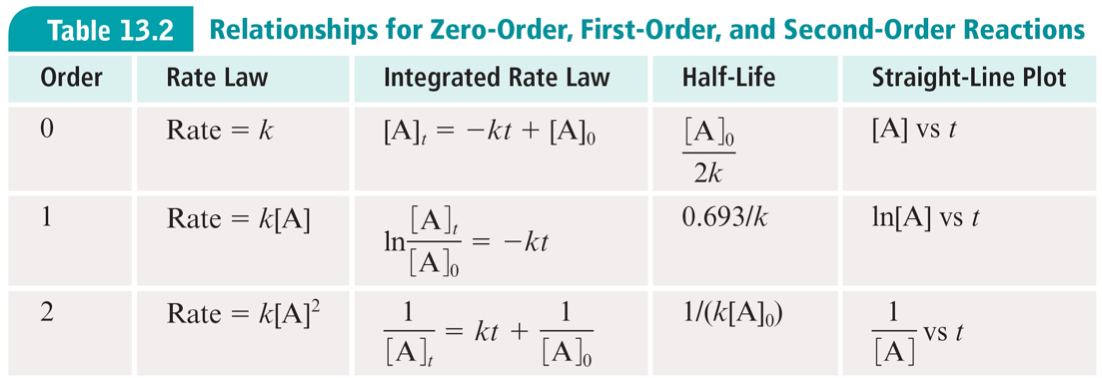
First Order Kinetics:
\[\ln(A)=\ln(A_0)-kt
\]
Second Order Kinetics:
\[\frac{1}{A}-\frac1{A_0}=k t
\]
The Depression of Freezing Point
\[\Delta T=K_f\times b\times i
\]
Calculate the freezing point of a \(0.09500\ m\) aqueous solution of glucose. The molal freezing-point-depression constant of water is \(1.86 °C/m\).
\[ans=1.86\times0.095\times 1 \]\(b\) 为摩尔浓度
\(i\) 是电离出的离子的个数(非电解质 \(i=1\))


 浙公网安备 33010602011771号
浙公网安备 33010602011771号